
Copper pops up everywhere! This surprising element appears in a cornucopia of modern products. Engineers and artists choose copper for a bundle of different uses. Its natural abilities make it an obvious choice for almost any purpose.
Mankind first discovered smelting with copper. In many ways, the history of humanity progressed in tandem with our uses of copper. It's no wonder copper has earned the nickname, “The Eternal Metal.”

Copper doesn't confine itself to stuffy history books, though! This multipurpose metal turns up in many unexpected places. We've collected some fun facts about copper to showcase the endless areas where copper appears.
In our investigation of interesting facts about copper, we'll cover a variety of areas:
- Facts about elemental copper
- Copper wires
- The Statue of Liberty
- The American penny
- How copper smelting changed history
- Bronze facts
- How brass created the trumpet
- Copper computer circuits
- How copper keeps you alive
Throughout our trip into the world of random copper facts, we'll see how copper has lent itself to our needs, both big and small. Whether it's the skin of the Statue of Liberty or a miniscule computer circuit, copper is great at doing what it does!
An Elemental Introduction
To begin, let's learn some fun facts about copper chemistry. Don't worry if you snoozed through science class in high school — we won't overwork your neurons!

Copper's name on the period table is Cu. This comes from the Latin word cuprum, which also gives us the word copper. Cuprum in turns comes from the Greek word Kupros, the name of the island of Cyprus in the Mediterranean Sea, where much of the ancient world’s copper ore came from Its atomic number is 29, which means it has 29 protons in its atomic nucleus.
Copper reacts with the air, but not with water, and aside from some rare isotopes, it’s stable and non-radioactive.
Along with gold, it’s the only naturally-occuring metal with a distinct color of its own. While most other metals are silver or grey, copper’s reddish tinge is instantly recognisable. When left exposed to oxygen, copper develops a protective patina, in contrast to iron, which rusts and degrades.
The only two metals that are used more widely than copper are aluminum and iron. Most copper reserves contain low-grade ore combined with sulfur and other elements, so it has to be extracted with a complex process involving heat (smelting) or a chemical reaction, such as leaching.
Most of the world’s copper production occurs in South America, where Chile produces twice as much copper per year as its nearest competitor, Peru. But China, the U.S., Russia, Australia, and Zambia are among the top 10 producers, showing that copper reserves are widely distrubuted on Planet Earth. Over 97% of the copper that’s been processed was mined after 1900.
Will we ever run out of copper? In theory, yes, because copper is a natural resource found in finite quantities. But because copper is reusable, there’s less concern over peak copper than there is over peak oil. New reserves are still being discovered, keeping copper prices relatively stable.
Copper is also used in dozens of alloys, increasing its versatility. Bronze statues, brass instruments, and cupronickel coinage are all made up of copper and one or more metals, such as tin and zinc.
Copper's atomic properties contribute to its natural, multi-purpose abilities. Copper’s all-around usefulness becomes clear when investigating its role in wiring.
Copper Wires and the Electromagnet
In 1824, the British scientist William Sturgeon conducted an experiment. He wrapped bare copper wire around a horseshoe-shaped piece of iron and ran an electrical current through the wiring. The iron became magnetized. The invention of this first electromagnet using copper wire soon took civilization to the next level.
Copper electrical wiring and electromagnets contributed to the invention of the telegraph and, later, the telephone. This same technology led to motors, generators and the modern power plant. But what made copper the best candidate for wires?
Copper lends itself well to reformation and it boasts a high malleability. This lets it easily reshape into thin sheets. Copper also maintains high ductility, which permits stretching. This property explains copper’s popularity as a top choice for wires.
Copper's role in wiring also showcases its role as an electrical and thermal conductor. Copper's high electrical conductivity means it doesn't resist the flow of electrician current. Its low thermal conductivity also makes it perfect for wires. Copper doesn't transfer heat. This makes it a top choice for many products like computer circuitry and electrical wires.

Copper wiring remains an industry standard. If your took all the copper wire from your car, it would weigh 50 pounds. Copper wires appear in your TV, computer, kitchen appliances, cell phones and basically every other electronic device.
Beyond its scientific uses, copper also shows up in art. Its malleability makes a prime choice for sculptures. Perhaps the most notable example is the Statue of Liberty.
The Statue of Liberty
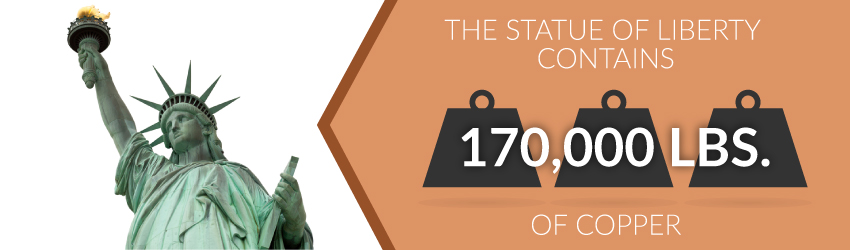
Copper needs no better spokesperson than Lady Liberty! From her feet to her torch, this 151-foot statue sports 179,000 pounds of copper. The majority of this copper forms her outer casing. Hewn to resemble her skin and robes, these copper sheets provide Lady Liberty’s trademark green color. But did you know that she's changed color over the years?
When the Statue of Liberty was inaugurated in 1886, she looked a little different. Just like a copper penny, Lady Liberty used to appear dark brown. Over time, she changed color. But where did she gain her chameleon-like powers? From copper, of course!
Ever discovered an old penny and wondered why it seems green? Well, the same science that transformed your penny also affected the Statue of Liberty. When copper reacts to oxygen and water, it undergoes oxidation. Over time, exposure to the elements causes a layer of green copper carbonate to grow on the copper surface. This process gives copper statues their famous green shade, known as a patina.
Don't worry about the patina weakening the statue, though — architects choose copper because of its strength. Unlike other chemical reactions, the patina doesn't corrode the copper. Instead, the patina enhances the copper's strength by creating a buffer layer to shield copper from other elemental factors.
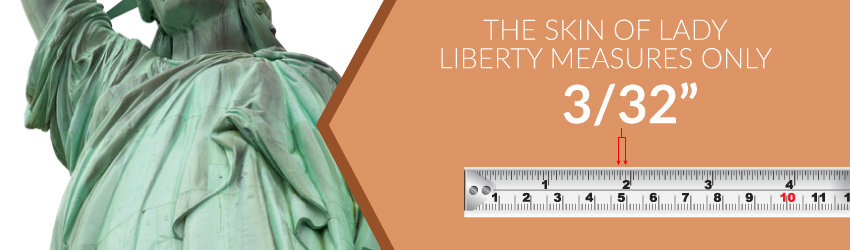
Copper is one of nature's strongest elements. In fact, the skin of Lady Liberty only measures the width of 3/32 of an inch. Picture two pennies pressed together. Although, the statue's weight mostly falls on its steel beam infrastructure, copper stands as the real star of the show.
When French designer Frédéric Auguste Bartholdi needed a material pliable enough to replicate ripples in fabric, but also strong enough to stand the test of time, he chose copper. 130 years later, it looks like he was right. Copper has lived up to the task!
The American Penny
Copper maintains a link to classic American iconography. From the colossal Statue of Liberty to the humble American penny, copper shows up everywhere. These two roles showcase copper’s association with everything America.
The history of the copper penny stretches back to 1787. This first penny, designed by Benjamin Franklin, was minted entirely from copper. Known as the Fugio Cent, this penny contains some curious imagery.
On the heads side, a sun dial, flanked by the Latin word Fugio, appears above the slogan "Mind Your Business." Instead of this coin telling you off, it actually forms a rebus, or pictogram puzzle. The sun dial represents time, and "Fugio" means "I flee," so the message reads, "Time flees, so do your work."
This type of cryptic advice bears the hallmark of Benjamin Franklin. After all, he did recommend consuming an apple every day to ward off medical professionals.
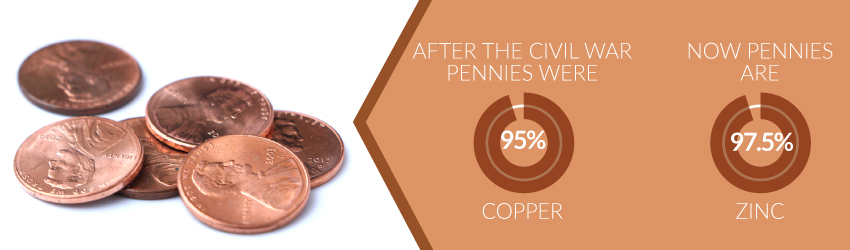
After the Fugio penny came the Flying Eagle cent in 1856. The image on this coin simply featured an eagle. The coin's composition became 88% copper and 12% nickel. It seemed that the penny's trademark ingredient was losing out, but in 1864, it made a brazen comeback. After the Civil War, the penny boasted an impressive 95% copper composition rate.
Our modern penny design emerged in 1909. To honor the centennial of Abraham Lincoln’s birthday, the mint emblazed the penny with his now-famous profile. The Lincoln penny also broke new ground by spearheading the phrase, now most synonymous with American currency, “In God We Trust.”
Unfortunately, the relationship between copper and the penny underwent some strain in 1983. Every penny minted after this transitional year bears a composition rate of 97.5% zinc. Copper only remains the thin layer covering the coin.
If you're curious whether your penny's composition boasts the classic copper recipe, there's a simple test available. Take your penny and flick it into the air. Let it drop on a hard surface like your floor or kitchen countertop. Listen to the sound it makes. If you hear a light and airy ring, it's copper. If the penny lands with a thud, sorry — it's a recent zinc penny. The crisp sound of copper makes it a prime choice for musical instruments like xylophones or cymbals.
The copper pennies also boast a low toxicity, unlike their zinc counterparts. In fact, it's quite dangerous to leave zinc pennies laying around your house. If your dog accidentally ingests a zinc penny, it may suffer from zinc poisoning and possibly die. If your dog ever mistakes a penny for food, go to the vet right away.
Sometimes, it's just better to keep it copper. However, copper usually does find work teaming up with other metals. It's a top player in the alloy game.
Metals to Trumpets: The Work of Copper Alloys
Copper loves collaborations! When copper teams up with other metals, magic happens. The process of combining metals creates metal alloys. Some of the most famous copper alloys include bronze and brass.
These two super-groups shaped the success of mankind. Without the technology of metal alloys, humanity might still be stuck in the Stone Age!
Forget about brass trumpets or bronze Olympic medals. Without metallurgy, a sharpened stone and spear might still represent "high-tech" tools to us. Once we melted metal, history cracked wide open and we've never looked back.
How Smelting Forged Our Future
Our ancestors had it rough. Before we learned to melt metal, all activity required found objects. Sharpened stones, antlers or bones typify the tools of this era. The discovery of melting copper to create metal tools first occurred in 5000 BC in Serbia. This discovery marks the passing of man from the Stone Age into the Copper Age.
In the millenniums to come, more instances of copper smelting began to emerge. Copper tools let people improve their productivity in hunting and, eventually, farming. The shift towards agriculture occurred gradually, but the discovery of smelting sped up development.
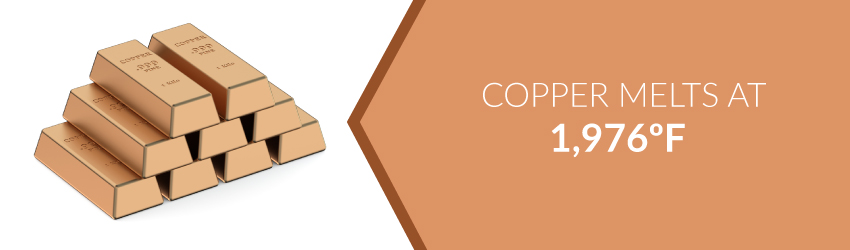
Copper possesses a relatively low melting point of 1,976°F. This allowed its easy extraction from the earth in ancient times. Freshly mined copper display a red-orange color. It appears as a pure metal in nature, and was easily extracted using elementary smelting techniques.
Early copper smelting techniques varied across the ancient world. Archeologists believe one type of copper extraction involved crushing the ore, sprinkling the copper dust over charcoal in a pit, covering the pit with turf, pumping air into kiln with an animal skin bellows and then removing the coagulated, cooled copper.
Copper combines well with other metals. The chemical structure of copper allows it to fuse effectively with metals like zinc or tin. Moreover, when copper blends with these metals, the fusion creates a stronger super-product. The pivotal discovery of mixing copper and tin led to the birth of bronze. This revelation occurred in 2900 BC in the Near East, and kicked off the Bronze Age.
Mankind boarded the fast-track from that point. Formidable bronze tools allowed for improved agriculture, which led to larger permanent population centers. These settlements evolved into the first cities. As the inhabitants diversified their work, social hierarchy emerged. The invention of writing encouraged business transactions, contracts and laws. These early empires used their bronze weapons to conquer each other, which further spread their technology. War created the need for further advancements to compete for survival. Next, mankind created iron, leading to the Iron Age, and so on.
As you can see, our history maintains a very old relationship with copper. Without it, where would we be now?
Copper at Sea
One of the most surprising uses of copper may be in shipbuilding. Throughout history, some form of metal sheathing has been used to protect vessels at sea. The ancient Greeks relied on lead plates, but beginning in the 1700s, copper sheating became the material of choice for shipbuilders.
Wooden ships were susceptible to any number of issues, from barnacles that impacted the handling of the ship, to shipworms that bored through wood. Even salt water could cause corrosion. While lead sheaths offered some protection, they reacted poorly with iron ship parts, in a process called galvanic corrosion. Copper turned out to be a more suitable alternative.
The Royal Navy was the first fleet to use copper sheathing, starting in the 1750s. Not only did the copper deter the growth of weeds and barnacles, but it reacted with the water to create a thin film made of copper oxychloride, which acts as a fungicide.
Hundreds of British ships were outfitted with copper sheathing during the war against the American colonies, and the practice was adopted by other navies as well. Tipu Sultan, leader of the Kingdom of Mysore in modern-day India, outfitted his navy with copper-bottomed ships.
Merchant ships also began to incorporate copper sheathing, particularly those that sailed in warmer ocean waters, where damage from bioorganisms was more of a concern.
Copper sheathing still presented some issues, though. Like lead, it reacted with the iron bolts in the hull, requiring those bolts to be relaced with a zinc and copper alloy.
Also, it was expensive. Another alternative came along with the development of Muntz metal, an alloy made of 40% zinc and 60% copper patented by George Muntz in 1832.
Its most famous application is on the hull of the Cutty Sark, a clipper ship built in Scotland in 1869 that primarily traded in tea and wool. It’s now displayed at the Royal Museums in Greenwich, just outside of London, where you can touch the copper hull of the ship yourself!
What Happened to Bronze?
Sure, bronze changed the course of human history, but what has it done lately? A lot, actually! Bronze remains a choice material for many modern-day products. Despite the prevalence of other alloys these days, some aspects of bronze make it a perfect metal for many items.
- Sculptures: Bronze maintains a relatively low melting point. This advantage allows sculptors and artisans a perfect metal to forge into statues. Most artists don't have access to super-high-temperature forges, so bronze's modest requirements for liquefaction make it a prime candidate for non-industrial craftsmen.
- Medals: The bronze medal isn't the most prestigious, but it's still a major accomplishment! If you're an Olympian athlete, you'll likely love your bronze medal even more than a silver one. A 1995 psychological study showed that, while silver medal winners felt like losers, bronze winners were much happier having won anything.
- Instruments: Bronze sounds great! Many musical instruments employ bronze to ring out their notes. Many brands of guitar strings use bronze as the main component. Drum cymbals also use bronze as the go-to compound. It doesn't break when struck, and emits a clear sound. Balinese gamelan orchestras also use bronze xylophones to make their famously unusual and beautiful music.
- Industrial Uses: Bronze's qualities make it a perfect candidate for many industrial purposes. It has a low friction point, so bronze appears in pistons and springs. Bronze is also an ingredient in flame-retardant hammers, because it never sparks when hit.
- Bronze Doorknobs: Copper demonstrates natural antibacterial properties. This aspect makes it a sanitary choice for doorknobs, banister railings and other public fixtures. Copper remains a scientific staple in the fight against infection. A 2011 study from Applied and Environmental Microbiology claims copper has a "contact killing" ability. This means that bacteria cannot survive on the exposed surface of copper. This renewed interest in copper's sanitary potential has led to use of copper in the fabric of antifungal socks.
Brass

Unlike the early arrival of bronze, brass came onto the scene much later. Like bronze, brass amalgamates copper with another metal. Except, instead of tin, copper teams up with zinc. Zinc alloys work a little differently than tin ones. Unlike tin, zinc has an incredibly high melting point of 788ºF. In fact, true zinc alloy brass wasn't made until the 18th century.
Earlier techniques skirted liquid zinc in a number of ways. Prior to modern smelting technology, a material called calamine was mixed with copper to produce a product similar to current-day brass. The Romans added low percentages of zinc to copper to create variously hued brass. The gold-like luster of this brass made it prized for jewelry, coins and helmets.
When Europeans finally invented higher-grade furnaces, brass gained popularity. Superior zinc extraction techniques gave brass the boost it needed. Brass soon found its way into many products. Even today, brass pops up in many everyday items.
Trumpets
There's nothing like a big brass band! The trademark sound comes directly from the instruments. Brass revolutionized the development of horn instruments. The emergence of horns, and especially the trumpet, shaped modern music. From mariachi to jazz, the brass trumpet gave various people a new language to express themselves. And that's something to toot your horn about!
Despite their brass makeover, though, trumpets aren't a recent invention. Metal trumpets dating from 1500 BC serve as our earliest examples. Bronze and silver trumpets even showed up in King Tut's tomb. Maybe people called the boy pharaoh “King Toot,” if the trumpets offer any indication.
Historically, trumpets served as religious and military items. The bugle offers a familiar equivalent. It wasn't until the Middle Ages that metallurgy improved enough to fashion musical trumpets. However, their role was limited due to their lack of valves.
When technology finally caught up, the trumpet became a dynamic and forceful instrument in European classical music. This chromatic brass trumpet had the ability to play any note. The modern trumpet was born.
As musical traditions shifted toward recordings and popular appeal, trumpet players like Louis Armstrong emerged. The foundational jazz of Miles Davis and Dizzy Gillespie helped bring the trumpet to the avant-garde side of modern music.
The trumpet demonstrates a truth about art and technology. Whenever technology improves, art finds a way to exploit those developments. Without the smelting breakthroughs of the 1800s, the trumpet would have remained an inferior bugle (no offence to bugles). The trumpet helped forge modern music. This artistic achievement rests on the technological advancement of copper. As technology continues to use copper, art will use those new discoveries to redefine artistic expression using the tools available.
The next step for copper appears to concern the computer. Naturally, the most impactful machine ever created uses copper. The metal that brought us out of the stone age also brought us into the digital age.
Copper in Computers
Copper never misses an opportunity to help out. Copper comes to the service of computers by enhancing the microchips. Copper aids the electrical and thermal transmissions within these chips. Copper's exceptional skills at conducting electricity without heating up make it a choice metal for circuits. This type of copper circuit also appears in cell phones and other electronic devices.
Copper demonstrates an omnipresent quality: It shows up everywhere! It even appears some places where you'd least expect, like your own body.
Copper in Your Body
Copper stands as an essential metal for bodily health. Along with iron, copper aids in maintaining normal metabolic processes. Many critical functions of your health owe their success to copper. But what exactly does copper do?
Copper reacts with proteins to create enzymes. These serve to spark biochemical processes. For example, copper acts as a catalyst in creating new cells. Tissue cells, blood cells and nerve cells all require copper to undergo creation. Copper plays a big role in metabolizing the myelin sheath that guards your nerves. Your thyroid requires copper to continue its normal function. The collagen in your bones owes a debt to copper, too.
Your heart and arteries use copper to maintain their vital role in your well-being. In fact, copper deficiency can lead to a greater risk of coronary heart disease. All around, copper holds up some key parts of your body.

The World Health Organization stresses the importance of consuming 1.3 mg of copper per day. Don't panic over your copper intake just yet, though! You likely consume the required amount without even noticing. However, it's wise to maintain awareness of your copper consumption.
Many delicious foods contain high levels of beneficial copper. The top picks include nuts, seeds, liver, chickpeas and oysters. Other nutrient-rich foods, like fish, meat and grains also boast a bunch of copper. Try to eat healthy and you'll end up absorbing the requisite amount of copper.
Copper in Medicine
In addition to being a necessary mineral in your diet, copper has other uses in health and medicine. Its anti-microbial qualities have been shown to reduce the spread of infection in hospitals when it’s used as a coating on bed rails, call buttons, stethoscopes, and other devices.
One design firm is even incorporating copper into its mobile Covid-19 testing units in an attempt to reduce transmission of the coronavirus.
Copper is also commonly used as an IUD to prevent pregnancy. It’s been shown to be 99% effective as a birth control method and can last for up to 10 years. How does it work? Essentially, the copper wire causes an inflammatory response in the uterus that interferes with the movement of sperm.
While copper IUDs aren’t suitable for everyone, they might be a good option if you want to avoid the side effects associated with hormonal IUDs.
And in Chile, copper socks were used to treat foot infections among the 33 miners who were stuck underground for over two months in 2010. Halfway through their ordeal, they were sent socks that contained iron oxide fibers, which acted as an anti-fungal agent to protect their skin.
We Have Used Copper For 10,000 Years
The latest research suggests that copper has been used by humans for at least 10,000 years and perhaps even longer than this. In 1991, a team of researchers discovered Otzi, the glacier mummy, hidden deep in the Otztal Alps. The mummy dated back to 3300 BCE and sported an ax that consisted of 99.6 percent copper. It seems even back then metal workers understood the importance of purity when creating copper objects and it’s a lesson that is still important today. That’s why at CopperSmith all the products we sell have the highest standard of copper purity. This ensures that any copper product is long lasting and isn’t beaten by the hands of time.
There are other examples of copper being used throughout history too. For instance, studies have suggested that Neolithic communities were using copper as an alternative to the typical stone tools favored by other civilisations. These communities date back to approximately 8,000 BC. There are many copper items dating back to medieval times that are preserved in near pristine condition. This is another one of the reasons why copper is still a popular choice for everything from currency to home design.
The ancient Egyptians also used copper. They believed that the material was sacred and granted any wearer with magical gifts. The idea that copper would possess healing qualities isn’t as far-fetched as it seems, as we’ll explain further down in this article. The Egyptians are also believed to be the first people to discover the antibacterial properties of copper. All of our copper products including our range hoods have the same antibacterial properties, ensuring that they are a perfect addition for any kitchen. You can get a quote for any copper product we sell now.
The name ‘copper’ is also believed to have originated with the Romans. They referred to the metal as ‘aes cyprium.’ This translates to ‘ore from Cyprus.’ During ancient times, this was where the majority of the metal was sourced from.
Copper And The Final Frontier
Copper products have been launched into space. It is arguably fitting that copper was chosen when designing a capital that would be sent out to the far corners of space. A copper time capsule was launched by NASA in 1977. The aptly named ‘Voyager Golden Records’ consisted of two gold plated phonograph records. These contained various images and sounds from earth. Overall there were:
- 115 images
- Greetings in 55 languages
- Natural sounds such as rain, thunder, birds, whales
The records are intended to provide a complete story of our world to future space farmers as well as extra-terrestrials. They include the inscription:
“To the makers of music, all worlds, all times.” As you may have guessed, copper was chosen to ensure that these data disks would remain intact for billions of years. It is quite likely that the disks will continue to exist, even after humanity is long gone from the world.
The records are currently the farthest human-made object away from earth. They have also been depicted several times in works of science fiction including The X-Files, Starman, and Fafner in the Azure. It is entirely possible that the gold-plated copper records are how we one day make first contact with extraterrestrial life.
Copper Is Included In Virtually All Gold Products
Have you ever seen a product that is advertised as being ‘pure gold.’ As it turns out, that’s unlikely to be the case. The reason for this is that gold is incredibly soft in its purest form. A stick of butter has the closest level of firmness to a slab of pure gold. You could quite easily carve your name in it. This is why even ‘pure-gold’ products will be made with a percentage of copper. Even 24-carat gold products will include a small amount of copper content. Without this, gold products would change form all too easily.
Any jewelry that you buy, whether it’s a watch, a necklace or a ring, will probably include some copper to strengthen the product. The only metals where amalgamation is exclusive are silver and copper. These are strong enough to stand on their own without the need to add any other metals.
The strength of copper is another reason why we favor it when designing kitchen and bathroom products. Any copper item we sell will last for decades.
It’s also interesting to note that compounding gold with metals like copper was banned in Europe as late as the 19th century. This has of course since been rectified and copper is used in almost every gold product that you can purchase today.
Our Cavalcade of Copper Facts Concludes
As you've seen, copper fulfills a diverse array of purposes. Its endless potential to solve our needs will certainly continue into the future. From the first metal tools of the Copper Age to the circuitry of the Digital Age, copper has held humanity's hand and guided our path.
No matter where time will take us, copper will be there. Whatever tools of the future we forge, copper will play a role. We only need to wait to see what copper will do next. An even greater number of interesting facts about copper will entertain our future descendants. As copper claims its place in the generations to come, more random copper facts will emerge!
At CopperSmith, we appreciate the history of copper. We create our products with a knowledge and respect for this durable and multi-purpose metal. Check out our website to see how we've taken copper to the next level of application and artistry. From our kitchenware to our beautiful copper counter tabletops, we know you'll find a product you love, including copper kitchen tables, sinks, copper vent hoods and designer fire pits.






