Have a customer looking for a particular copper farmhouse sink design or color? Contact us for free design help & wholesale pricing.
About Copper Patinas

Copper is an extremely durable metal that withstands corrosion and retains its functional properties for up to 90 years. And once it’s reached the end of its useful life, at least 90 percent of it can be recycled for reuse.
Whatever stage of its life, copper’s appearance changes when it is exposed to the elements. A colored film called a patina is formed on the copper surface. The different patinas are the reaction of copper with moisture oxygen and sulfur-based compounds. The patina acts as a barrier protecting the copper underneath against corrosion.
In this blog, we look at various colors and shades of patinas, the timelines for the color changes and why, over enough time, copper becomes bright green.
How do you say “patina”?

First, the term patina isn’t a word you hear every day, so if you’re just encountering it for the first time, you might be wondering how to pronounce it.
Is it PAT-en-ah or pa-TEE-nah? Mirriam-Webster offers both pronounciations, but pa-TEE-nah seems to be the most common pronunciation these days, at least in the U.S.
The etymology of the word dates back to the 1700s, when Italians derived the word from the Latin for “pan” or “shallow dish”. This suggests that it was originally a reference to the film that appears on copper cookware, but over the next few centuries, it expanded to include the protective film or gloss on other polished metals and even wood.
It can also refer to a more abstract “aura” surrounding a person, place, or thing. You can learn more about the history of the word on the Mirriam-Webster Word of the Day Podcast. For now, let’s get on to the science.

Why Copper Changes Its Color

In the first few weeks of exposure, especially in a humid climate or in places with frequent rainfall, the surface of copper can dramatically change colors with the metal displaying streaks of pink, orange, red interwoven with yellow, blue, green and purple. With continued exposure to moisture and other forces, the initial interference colors will give way a solid russet brown that is often called an oxidized or statuary finish.
In some copper mills, due to variable fabrication techniques, the surface of the flat or coiled sheet stock may be coated with a thin film of anti-stain oil. This film may produce a black or dark purple surface coloration shortly after it is installed and exposed to weather. However, this is just a temporary color change that will be wash off from rain after a short time and the natural weathering process of the copper will continue.
In coastal regions or heavy-industrial areas, the natural patina typically forms within five to seven years. In the country and rural areas, where the level of sulfur dioxide in the atmosphere is relatively low, the patina formation takes 10 to 14 years to attain a dominant stage. In dry regions, the basic patina may never be formed because of the extremely low atmospheric moisture.
Meanwhile, exposed horizontal copper surfaces develop their patinas faster than sloping surfaces. And patinas form faster on sloping surfaces compared with those on vertical surfaces. In each instance, the key factor affecting the patina is the amount of time that moisture and sulfur compounds interact with the exposed surface.
Three kinds of films form on exposed copper surfaces: sulfate, sulfide and progressive oxide. The sulfide and oxide films don’t provide much resistance to corrosion while the sulfate film provides strong resistance to all types of atmospheric corrosion as soon as it is fully formed. Therefore, it enhances the durability and lifespan of copper roofing tiles and sheets.
Timelines for Color changes
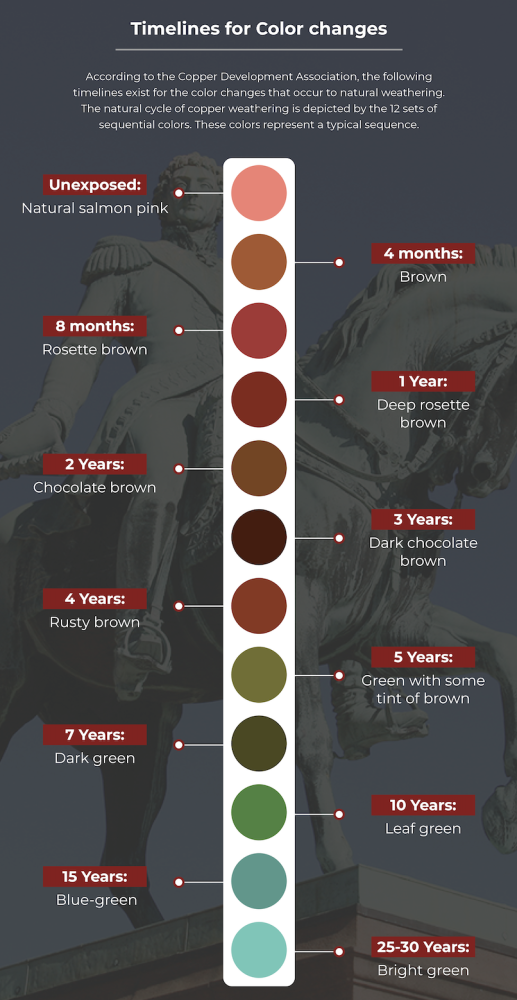
According to the Copper Development Association, the following timelines exist for the color changes that occur to natural weathering. The natural cycle of copper weathering is depicted by the 12 sets of sequential colors. These colors represent a typical sequence.
However, the weathering of any internal or exterior copper structure depends on the residual lubricants, environmental factors, and the orientation after installation. Every copper surface will attain a final patina color within its local environment and thereafter no more weathering or color changes will occur.
- Unexposed: Natural salmon pink
- 4 months: Brown
- 8 months: Rosette brown
- 1 Year: Deep rosette brown
- 2 Years: Chocolate brown
- 3 Years: Dark chocolate brown
- 4 Years: Rusty brown
- 5 Years: Green with some tint of brown
- 7 Years: Dark green
- 10 Years: Leaf green
- 15 Years: Blue-green
- 25-30 Years: Bright green
Using Chemicals to Creating Different Copper Patinas

Due to the time it takes for copper to develop different patinas through weathering; architects, engineers and home designers know how to use chemicals to speed up this process and obtain the type of copper patina they desire.
Treating copper with chemicals is an art and the outcome depends on surface preparation, temperature, time, humidity and other factors. Virtually all natural patinas and several other colors can be produced on pure and copper based alloys with the aid of chemical conversion coatings. The conversion coatings speed up the natural weathering effect that occurs from exposure to the elements. Some coatings are also added to preserve the original color of the copper surface and prevent it from developing other patinas through natural weathering
Chemical coloring is basically achieved through the use of acid sulfate treatments or acid chloride treatments. However, due to several factors, patinas produced by chemical induction are usually prone to undesirable conditions such as poor adhesion, uneven coloration over a large surface area and excessive staining of neighboring materials. Therefore, these drawbacks should be carefully considered before applying chemicals.

Why copper?
Copper is a popular choice for building materials and home decor due to its appearance, but there are plenty of other reasons to choose copper too.
Long lasting
The fact that copper has been used as a construction material for centuries should give you some idea of its longevity. Some studies suggest that copper roofs can last for up to 1000 years, and in rural environments, they corrode at .4mm or less every 200 years.
While that doesn’t mean a copper roof will never have to be repaired or changed, it’s more likely that the underlying materials will be the issue, not the copper itself.
A large part of copper’s durability is due to its patina, which protects it from the elements and resists surface corrosion in many environmental conditions. Want more information on how copper interacts with kitchen products? Check out this blog post on copper sinks.
Lightweight and low maintenance
Copper is relatively lightweight, reducing the load on the structure when compared to other building materials, such as roofing tiles. Copper cladding is even lighter, and may be as little as .7mm thick. It’s often used on the exteriors of buildings as a protective layer over another material, meaing you don’t need to use a lot of copper to develop a patina on the surface.
Copper has a high melting point and 40% lower thermal expansion than zinc or lead, which means it’s unlikely to expand or contract under normal conditions. It can also be used in both “warm” and “cold” roof ventilation techniques, and requires very little maintenance after installation.
Green in more ways than one
Finally, copper is prized for its sustainability, and is increasingly being incorporated into “green” building designs. Copper is 100% recyclable and it can be reused again and again, unlike many metals, which degrade each time they are recycled. More than half of the copper that’s used in construction is recycled, using only 20% of the energy of primary production.
Additionally, the use of copper in architecture can have other benefits, including lower energy costs due to its thermal efficiency. The Biodesign Institute C at Arizona State University is “wrapped in a screen of thousands of perforated, naturally finished copper panels that contrubute to the building’s energy efficiency and sustainability,” helping it achieve a LEED Platinum certification.
Copper is also biostatic, which means that it resists the growth of bacteria. While too much copper in the soil can be toxic to plants, a moderate amount of copper fertilizer is good for them.
Other Finishes
One last thing that can impact the look and feel of your copper decor is the finishing. While this is related to the patina, there are additional components that affect the surface of the material.
Mechanical treatments
Mechanical treatments use physical processes to roll, buffer, or grind the surface, covering up any imperfections in the fabrication process. You can choose a rugged, unfinished look, a matte finish that creates a dull surface, or a polished and buffed finish for a bright and shiny surface.
Fine, medium, and coarse satin finishes provide a smooth surface to varying degrees, while hand-rubbed finishes use either a wire brush or a woven pad to smooth down the copper.
Nondirectional textured surfaces are made by blasting sand or shot at the material. You can also choose patterned surfaces made by pressing the copper between two design rolls.
As you can see, the kinds of textures you can achieve with a copper surface are nearly infinite!
Coatings
Another thing to consider is whether you want to apply a protective coating to your copper. While coatings aren’t often practical for exterior use, since they require more maintenance than a patina, they can be a good option if you want to retain the shiny look of natural copper.
Coatings include clear organic coatings such as acrylic, epoxy, and silicone, which are transparent and allow the natural color to show through, or opaque coatings, such as paints or enamels, which are used when the original copper color isn’t desired.
Other options are oils and waxes, which can protect the copper from moisture and retain its color longer, slowing down the transition from black to green. These coatings are useful for decorative copper features, such as statues or gates, but can also be applied to copper roofs.
Coating for Preservation of Original Tones
Meanwhile, preserving the original salmon pink color or the polished gold tones on the copper and bronze or brass surfaces can be achieved by using clear protective coatings that act as a barrier to natural weathering and prevent the oxidation of the copper surface. However, please note that virtually all coatings will wear out and get degraded over time. So these coatings will need to be maintained through stripping and replacement.
How Does CopperSmith Use Copper Patina?
At CopperSmith, we understand that many people enjoy the look of patina on their copper products. It gives a beautiful aged look and acts as a barrier to prevent corrosion, so there are benefits both in terms of aesthetics and functionality. However, since the process of developing a patina can take roughly 30 years from the natural brown color of copper, it’s necessary to use chemicals in order to develop a patina.
Copper Bathtubs
A great example of this is our copper bathtubs. All of our copper products are made to order and can be customized to your exact liking. For our bathtubs, we offer many different finish choices from aged brass to polished copper. However, one of the most popular styles we offer is copper verde. This creates a beautiful green patina shell around the bathtub in a decorative pattern.
In most cases, using chemical coloring to create a patina effect usually results in undesirable effects. For example, if the chemicals are spread too much, then it could lead to uneven coloration or staining of materials that weren’t intended to be affected. It could also affect the adhesion of the material and is a drawback that must be considered before choosing patina copper.
But in the case of our copper bathtubs, the patina is used only as a decorative element on the outside of the bathtub. As such, we can mitigate many of the downsides. What we’re left with is a beautiful decorative patina on the outside of our bathtubs. This can be applied to all of our bathtubs and isn’t limited to a handful of designs. This patina can also help to protect the design of the freestanding bathtub.
Copper Range Hoods
We also offer copper range hoods with patina finishes. While natural patina will change copper into a green color, chemical colorings allow us to mix different shades to create something completely unique.
For example, we can create a rustic fire or Tuscan copper patina that has the same shiny polished surface of a patina but has a different color that may be more aesthetically pleasing to some. The ability to change the patina also means it can fit better in your overall home decor style. When combined with our other customization options such as the crown profile, rivets, strap texture, and mount, we can offer completely bespoke copper products that are designed to meet your needs.
The copper patina on a range hood adds visual appeal to enhance its look. It adds a unique texture to the copper while also enhancing the colors. Since we can mix different shades, we can transform the copper to look like a vintage piece of metal, or we can give it a rustic look to suit the rest of your home. It’s highly customizable and gives you plenty of options.
The copper patina also adds a natural layer of protection to the surface of the range hood. Since it’s being used to exhaust air out of your home, the layer of patina acts as a barrier to prevent moisture, pollutants, and oxidation. This gives copper patina a functional use for range hoods and can help to extend its lifespan. ALways keep in mind you can do a copper recirulating hood as well.
Finally, copper patina means that your range hood will require less maintenance compared to other finishes. As mentioned already, the patina will protect the range hood from environmental factors, but it’ll also naturally maintain its shine without the need for regular cleaning and polishing. This keeps the patina looking attractive with minimal effort.
In short, copper patina on range hoods drastically improves its durability while also giving it a beautiful unique look that you can fully customize with CopperSmith. Check out these great kitchens with copper hoods.
Copper Fire Pits
Our copper fire pits offer pre-weathered finishes for people that want the typical green patina style. This can help to protect your fire pit against the elements, especially as it will typically be used outdoors. The layer of patina will shield it against moisture and other outside pollutants, helping it retain its color and durability.However, not everyone likes a green copper fire pit. To remedy this, CopperSmith offers a range of different patina styles and textures to suit all tastes. If you’re not a fan of the natural green patina, then we can create a patina with a completely different hue. We can pre-weather your fire pit to create a beautiful rustic patina of your liking. This will protect the fire pit while also helping it with the rest of your outdoor decor.
For more information on our copper products, click here.






